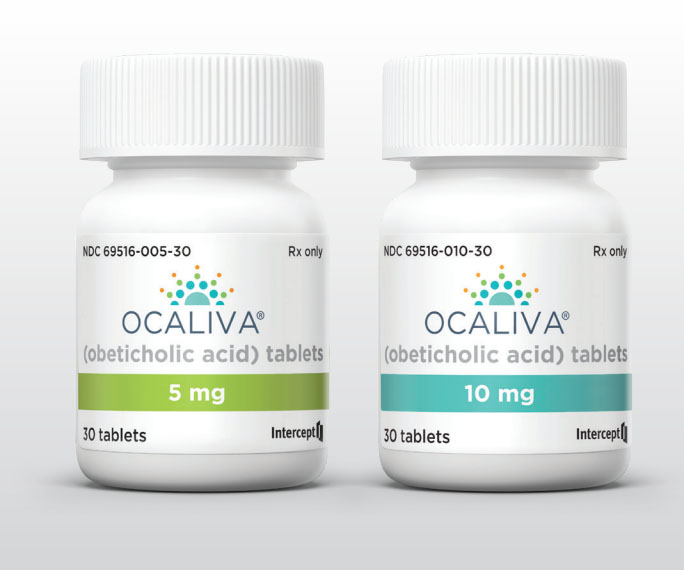
Liver drug benefits ‘not confirmed’, revoke authorisation EMA says
The European Medicines Agency’s (EMA) human medicines committee (CHMP) is recommending that the marketing authorisation for a liver disease drug be revoked because its benefits are no longer considered ...